No products in the cart.
Navigating FDA & USDA Regulations for Frozen Food Packaging
Why Compliance Matters for Frozen Food Packaging
Ensuring your frozen food packaging materials meet regulatory standards for food contact substances is important because, at its core, these global and FDA regulations protect the consumer from harm. Since the USDA and FDA regulations for frozen food packaging prevent harmful substances from contaminating food, the food won't pose a health risk to consumers. These food safety regulations ensure frozen fruits and foods are safe to eat and that the quality of the food doesn't degrade during storage regardless of its shelf life.


By complying with the food labeling requirements and other regulations set forth for the food industry, not only are you effectively avoiding litigation due to non-compliance, but you're helping build trust with your customers. Yes, the Food and Drug Administration sets proper packaging standards to protect the health of consumers, but adhering to these strict regulations plays a crucial role in your brand's reputation in the market.
If you're able to avoid giving your customers foodborne illnesses by ensuring compliance and therefore preventing contamination during the transportation, cooking, or freezing process, this is a well known example of the crucial role packaging plays in customer satisfaction.
Global Regulations for Food Contact Materials
Although this blog focuses primarily on the federal food regulations of the USDA and FDA, it's important to note that several food safety standards for food manufacturing and storage exist globally. From the European Union to South American regulations, here are some of the more popular regulations set for packaging manufacturers on a global scale. These apply to manufacturers of all sizes in these markets, including those that supply and package frozen food for consumers.
European Union No 10/2011
Commonly referred to as the Plastics Regulation, this piece of EU legislation establishes safety standards for plastic packaging materials that come in contact with food. These food contact substances have specific requirements for composition, migration limits, and compliance testing. The regulation defines which monomers, additives, and other substances can be used in the plastic materials and has been amended three times to reflect new scientific assessments. Ultimately, it protects consumers from plastic contamination in their food.
MERCOSUR GMC n 3/92
This consumer safety regulation establishes protocols for food packaging manufacturers within MERCOSUR (Southern Common Market) countries, including Argentina, Brazil, Paraguay, and Uruguay. Like other safety regulations, this standard defines approved materials, additives, and monomers used in food packaging and requires mitigation testing and harmonization with other standards. MERCOSUR is updated regularly to reflect the latest advancements in food science, and always puts the safety of consumers first.
Brazil RDC n 91/2001
Brazil RDC Resolution No. 90/2001 is a regulation issues by ANVISA, and it establishes requirements for food contact substances within Brazil. Like the others, it sets specific migration limits, outlines testing and compliance requirements, and ensures that plastic materials do not alter the taste, odor, or composition of food products. If your company produces or sells food products in Brazil, you'll need to familiarize yourself with these food packaging requirements.
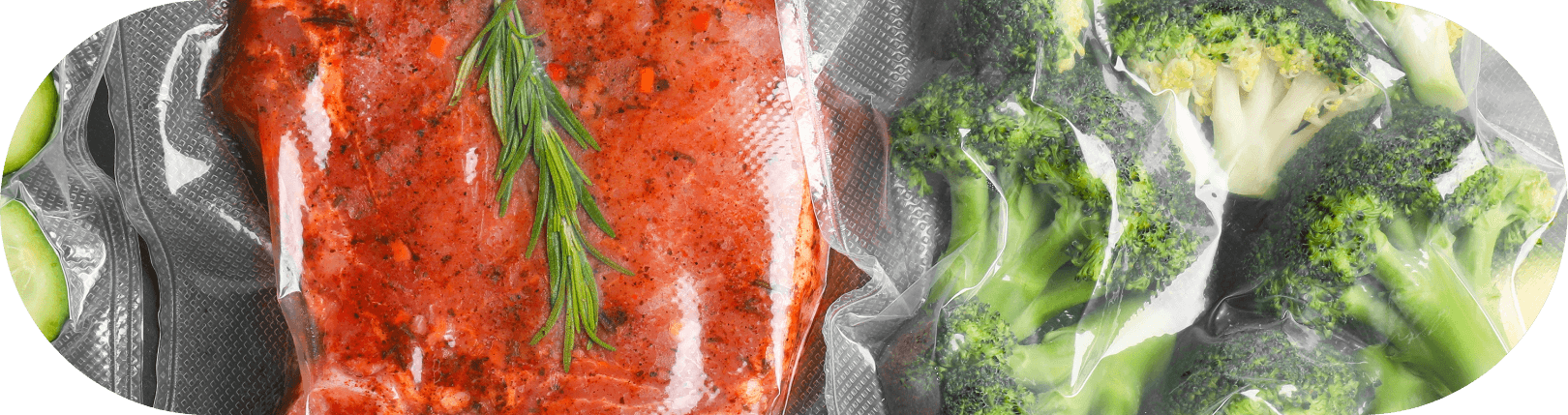
21 CFR: Understanding FDA Regulations for Frozen Food Packaging
In the United States, specifically, the FDA maintains Title 21 of the Code of Federal Regulations (21 CFR), which governs food and drug safety, and includes regulations for food contact materials. It is enforced by the FDA. Here, we'll discuss the key food safety sections relevant to food contact materials.
Key Sections Relevant to Food Contact Substances
21 CFR Part 174 – General provisions for indirect food additives, including basic safety requirements. It outlines Good Manufacturing Practices (GMPs) and if a material complies with an existing CFR regulation it doesn't require further FDA approval.
21 CFR Part 179 – This part of the FDA standard regulates the use of ionizing radiation (irradiation) during the production, processing, and handling of food-contact materials. Though it's not super applicable to packaging materials that come in contact with food, it is still worth mentioning.
21 CFR Part 175 – Establishes FDA regulations for adhesives and coatings used in most food packaging (e.g., can coatings, paper coatings). These regulations ensure that these packaging materials do not transfer harmful chemicals to food under normal conditions and during intended use.
21 CFR Part 180- Establishes regulations for food additives that are permitted for use by manufacturers in food processing. These substances are sometimes added to fresh and frozen food items, like canned food, to preserve freshness, improve flavor, and extend shelf life.
21 CFR Part 176 – Provides safety regulations for paper and paperboard that comes in contact with food. This includes paper-based packaging materials such as boxes, wrappers, bags, and takeout containers that come in direct or indirect contact with food.
21 CFR Part 181- Outlines the regulations for substances that are classified as Generally Recognized as Safe (GRAS) when used in food. This part provides the criteria for determining whether a substances qualifies as Generally Recognized as Safe or if it needs to go through the FDA approval process.
21 CFR Part 177 – This FDA safety regulation applies to plastic materials and resins. It establishes the requirements for the safety and suitability of polymers used in food-contact applications and lists approved substances and conditions for their use.
21 CFR Part 186 – Governs the use of polymers and other similar substances as indirect food additives in food packaging, processing, and handling. It ensures that polymers used in food-contact applications are safe and comply with FDA standards for migration limits and material safety.
21 CFR Part 178 – Lists food-contact additives, such as antioxidants and stabilizers for packaging, and provides regulations for them. Part 178 includes substances used in the production of food-contact materials such as plastics, coatings, adhesives, and paperboard, and ultimately focuses on preventing contamination.
21 CFR Part 189- This outlines a comprehensive list of food ingredients that are prohibited from being used in human food because of their potential to harm human health. This is part of the FDA's broader effort to ensure the safety of the food supply and protect consumers from contamination and dangerous ingredients or chemicals.
Key Packaging Considerations for FDA & USDA Compliance
If you're currently designing or manufacturing packaging for food manufacturers, here's how the USDA and FDA regulations impact packaging design and manufacturing.
Steps to Ensure FDA Compliance
Ensuring that your food packaging gets FDA approval is essential for consumer safety and the compliance of your company. Here's a quick checklist that can help you navigate the regulatory process effectively:
By following these steps, you can ensure that your packaging materials meet the requirements outlined by the FDA. Additionally, the food itself should be free from harmful additives and the packaging may need to include nutrition labeling, a breakdown of net quantity, nutritional content, information about the packaging's environmental impact, nutrition facts, or other information.

Steps to Ensure Your Frozen Food Packaging is Compliant
When it comes to ensuring your packaging materials meet the standards set forth for both direct and indirect food contact applications, there are a few things you can do:
Partner with an Experienced Supplier
By partnering with an experienced supplier, they'll guide you towards using materials that don't require FDA approval, ensuring compliance and product integrity.
Conduct Migration and Durability Testing
As discussed in the previous section, you should be testing your products for migration and durability. Additional testing for heavy metals, especially in high exposure applications, can also help.
Ensure Labels meet FDA and USDA Standards
Finally, you need to make sure your labels meet FDA and USDA standards; they should include specific labeling requirements like nutritional content or how to store your product for extended periods of time.


How CarePac Helps You Meet FDA Approval
At CarePac, we understand the importance of compliance with FDA and USDA regulations in ensuring the safety and quality of food products. Our custom flexible barrier packaging solutions are meticulously designed to align with all relevant regulatory standards, offering both protection and compliance for businesses in the food industry. If you're seeking FDA approval, we can help.
If you’re looking for FDA-compliant, USDA-approved packaging solutions tailored to your business’s needs, CarePac is here to help. Whether you need custom designs for frozen foods or other specialized packaging requirements, our team is ready to guide you through the process and provide the best solution for your product.
Contact CarePac today to learn more about our compliant packaging solutions and how we can help your business stay ahead of regulatory requirements while preserving product quality and safety.
Food and Drug Administration Packaging FAQs:
Some requirements for packaging that's intended to be in freezer storage for extended amount of time include using material that's moisture resistant, durable, leakproof, and capable of protecting foods from freezer burn during storage.
FDA regulations for food packaging ensure the materials used for packaging do not create a health hazard or change the taste, smell, or composition of the food. They include 21CFR.
Let's Get Started
Made In
The USA
Full Pouch
Customization


